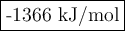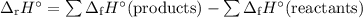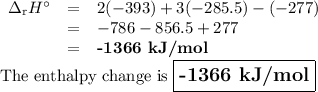Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 23.06.2019 07:30
How many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride
Answers: 1
You know the right answer?
The enthalpy of formation of c2h5oh ,co2 and h2o are -277 ,393 and -285.5 kj/mol calculate the entha...
Questions






Mathematics, 18.05.2021 18:40


Computers and Technology, 18.05.2021 18:40


Biology, 18.05.2021 18:40


Mathematics, 18.05.2021 18:40




History, 18.05.2021 18:40

Mathematics, 18.05.2021 18:40

Spanish, 18.05.2021 18:40


History, 18.05.2021 18:40