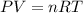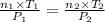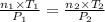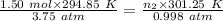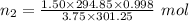Chemistry, 21.01.2020 19:31 steelemaddie20
Aglass container was initially charged with 1.50 mol of a gas sample at 3.75 atm and 21.7c. some of the gas was release as the temp was increased to 28.1c so the final pressure in the container was reduced to 0.998 atm. how many moles of the gas sample are present at the end?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

Chemistry, 23.06.2019 17:00
Using this reversible reaction, answer the questions below: n2o4 2no2 (colorless) (reddish-brown) -as the temperature increased, what happened to the n2o4 concentration? -was the formation of reactants or products favored by the addition of heat? -which reaction is exothermic? right to left or left to right? -if the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct? n2o4 2no2 + 14 kcal n2o4 2no2, hr = +14 kcal n2o4 + 14 kcal 2no2 n2o4 2no2, hr = -14 kcal
Answers: 1
You know the right answer?
Aglass container was initially charged with 1.50 mol of a gas sample at 3.75 atm and 21.7c. some of...
Questions

History, 27.04.2020 02:21


History, 27.04.2020 02:21



Mathematics, 27.04.2020 02:21


Mathematics, 27.04.2020 02:21

Mathematics, 27.04.2020 02:21


Mathematics, 27.04.2020 02:21


Mathematics, 27.04.2020 02:21

Mathematics, 27.04.2020 02:22


Chemistry, 27.04.2020 02:22

Mathematics, 27.04.2020 02:22

History, 27.04.2020 02:22


Mathematics, 27.04.2020 02:22