
Asolution is prepared by adding 49.3 ml concentrated hydrochloric acid and 19.5 ml concentrated nitric acid to 300 ml water. more water is added until the final volume is 1.00 l. calculate h and the ph for this solution. [hint: concentrated hcl is 38% hcl (by mass) and has a density of 1.19 g/ ml; concentrated hno3 is 70% hno3 (by mass) and has a density of 1.42 g/ml.]
[h+] =
oh- =
ph =

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
Asolution is prepared by adding 49.3 ml concentrated hydrochloric acid and 19.5 ml concentrated nitr...
Questions

Biology, 11.01.2021 18:10

Mathematics, 11.01.2021 18:10


Geography, 11.01.2021 18:10

Chemistry, 11.01.2021 18:10

Mathematics, 11.01.2021 18:10

Mathematics, 11.01.2021 18:10





Mathematics, 11.01.2021 18:10

Mathematics, 11.01.2021 18:10


Mathematics, 11.01.2021 18:10

History, 11.01.2021 18:10

Chemistry, 11.01.2021 18:10

Mathematics, 11.01.2021 18:10

Mathematics, 11.01.2021 18:10

Mathematics, 11.01.2021 18:10

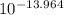 = [OH⁻] = 1.086x10⁻¹⁴ M
= [OH⁻] = 1.086x10⁻¹⁴ M

