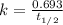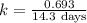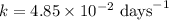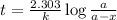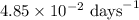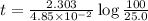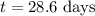Chemistry, 21.01.2020 21:31 jordantay208
The radioactive isotope 32p decays by first-order kinetics and has a half-life of 14.3 days. how long does it take for 75.0% of a sample of 32p to decay?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1


Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2
You know the right answer?
The radioactive isotope 32p decays by first-order kinetics and has a half-life of 14.3 days. how lon...
Questions

Social Studies, 01.10.2019 10:10


History, 01.10.2019 10:10

Computers and Technology, 01.10.2019 10:10


Social Studies, 01.10.2019 10:10

History, 01.10.2019 10:10





Biology, 01.10.2019 10:10






Mathematics, 01.10.2019 10:10