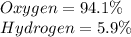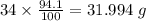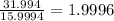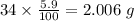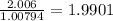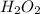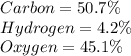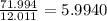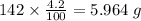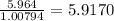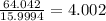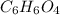Determine the molecular formula for each compound.
a) 94.1% oxygen and 5.9% hydrogen; molar mass = 34g
b) 50.7% carbon, 4.2% hydrogen, and 45.1% oxygen; molar mass = 142g
(would greatly appreciated if someone could explain the process, also use the correct amount of significant digits)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
Determine the molecular formula for each compound.
a) 94.1% oxygen and 5.9% hydrogen; molar...
a) 94.1% oxygen and 5.9% hydrogen; molar...
Questions


Computers and Technology, 16.12.2020 22:00

English, 16.12.2020 22:00


Mathematics, 16.12.2020 22:00


Mathematics, 16.12.2020 22:00

Health, 16.12.2020 22:00


Mathematics, 16.12.2020 22:00





English, 16.12.2020 22:00

Mathematics, 16.12.2020 22:00

Mathematics, 16.12.2020 22:00

Mathematics, 16.12.2020 22:00


 molecules.
molecules.