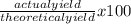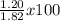Chemistry, 22.01.2020 00:31 jonmorton159
The actual yield of a product in a reaction was measured as 1.20 g. if the theoretical yield of the product for the reaction is 1.82 g, what is the percentage yield of the product?
a. 65.9%
b. 67.2%
c. 71.9%
d. 73.3%

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
The actual yield of a product in a reaction was measured as 1.20 g. if the theoretical yield of the...
Questions


Computers and Technology, 25.01.2020 20:31

Mathematics, 25.01.2020 20:31


History, 25.01.2020 20:31

Health, 25.01.2020 20:31


Physics, 25.01.2020 20:31




English, 25.01.2020 20:31


Mathematics, 25.01.2020 20:31


Biology, 25.01.2020 20:31




Biology, 25.01.2020 20:31