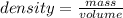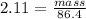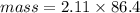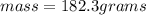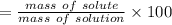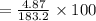Chemistry, 22.01.2020 04:31 ayoismeisjjjjuan
Asolution is made by dissolving 4.87 g of potassium nitrate in water to a final volume of 86.4 ml solution. what is the weight/weight % or percent by mass of the solute? (i got 5.64% but it said it was incorrect, and i can't figure it out? )

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 19:00
Imagine that a new planet is discovered with two moons of equal mass: moon a and moon b. the mass of the new planet is greater than the combined mass of its moons. moon a is farther away from the new planet than moon b. what is the planet's gravitational pull on moon a compared to the planet's gravitational pull on moon b? the planet's gravity repels moon a with a greater force than it repels moon b, which is why moon a is farther away. the gravitational pull on moon b is greater than on moon a because moon b is closer to the new planet than moon a. the gravitational pull on moon b is greater than on moon a because moon b is farther away from the new planet than moon a. the gravitational pull on moon a is the same as the gravitational pull on moon b because distance does not affect the planet's gravity.
Answers: 1

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3
You know the right answer?
Asolution is made by dissolving 4.87 g of potassium nitrate in water to a final volume of 86.4 ml so...
Questions

History, 16.09.2019 04:10

English, 16.09.2019 04:10




Mathematics, 16.09.2019 04:10





Mathematics, 16.09.2019 04:10

Mathematics, 16.09.2019 04:10

Mathematics, 16.09.2019 04:10

Mathematics, 16.09.2019 04:10




History, 16.09.2019 04:10

Mathematics, 16.09.2019 04:10