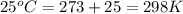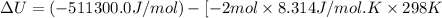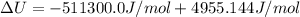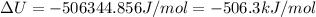Chemistry, 22.01.2020 04:31 george6871
The standard internal energy change for a reaction can be symbolized as δ u ∘ rxn or δ e ∘ rxn . for each reaction equation, calculate the energy change of the reaction at 25 ∘ c and 1.00 bar . sn ( s ) + 2 cl 2 ( g ) ⟶ sncl 4 ( l ) δ h ∘ rxn = − 511.3 kj/mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2

Chemistry, 23.06.2019 07:00
If you used the method of initial rates to obtain the order for no2, predict what reaction rates you would measure in the beginning of the reaction for initial concentrations of 0.200 m, 0.100 m, & 0.050 m no2.
Answers: 3
You know the right answer?
The standard internal energy change for a reaction can be symbolized as δ u ∘ rxn or δ e ∘ rxn . for...
Questions


English, 27.10.2020 05:20


History, 27.10.2020 05:20


Mathematics, 27.10.2020 05:20

Social Studies, 27.10.2020 05:20


Mathematics, 27.10.2020 05:30



English, 27.10.2020 05:30

English, 27.10.2020 05:30

Mathematics, 27.10.2020 05:30


English, 27.10.2020 05:30


Mathematics, 27.10.2020 05:30


Mathematics, 27.10.2020 05:30

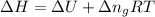
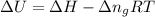
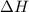 = change in enthalpy =
= change in enthalpy = 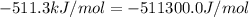
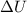 = change in internal energy = ?
= change in internal energy = ?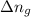 = change in moles
= change in moles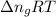 = 0
= 0