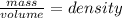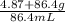Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Asolution is made by dissolving 4.87 g of potassium nitrate in water to a final volume of 86.4 ml so...
Questions

Mathematics, 26.02.2021 20:20

Mathematics, 26.02.2021 20:20


English, 26.02.2021 20:20

Mathematics, 26.02.2021 20:20


Social Studies, 26.02.2021 20:20

History, 26.02.2021 20:20





English, 26.02.2021 20:20


Law, 26.02.2021 20:20

Mathematics, 26.02.2021 20:20



History, 26.02.2021 20:20

Chemistry, 26.02.2021 20:20