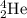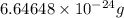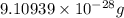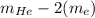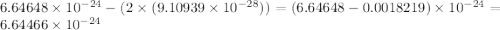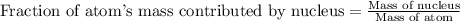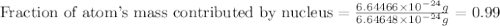Helium is the lightest noble gas and the second most abundant element (after hydrogen) in the universe. the mass of a helium−4 atom is 6.64648 × 10−24g, and each of its two electrons has a mass of 9.10939 × 10−28g. what fraction of this atom's mass is contributed by its nucleus?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
Helium is the lightest noble gas and the second most abundant element (after hydrogen) in the univer...
Questions



Mathematics, 07.06.2021 07:40

Mathematics, 07.06.2021 07:40

Mathematics, 07.06.2021 07:40

Mathematics, 07.06.2021 07:40


Mathematics, 07.06.2021 07:40

Social Studies, 07.06.2021 07:40

French, 07.06.2021 07:50




History, 07.06.2021 07:50




Mathematics, 07.06.2021 07:50

Chemistry, 07.06.2021 07:50

Mathematics, 07.06.2021 07:50