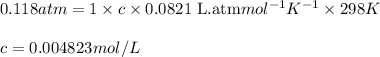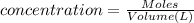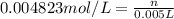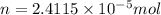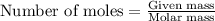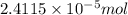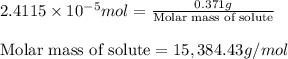Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1
You know the right answer?
An unknown protein are dissolved in enough solvent to make of solution. the osmotic pressure of this...
Questions

Mathematics, 14.12.2021 04:50

History, 14.12.2021 04:50


Mathematics, 14.12.2021 04:50


Mathematics, 14.12.2021 04:50



Computers and Technology, 14.12.2021 04:50







Arts, 14.12.2021 05:00

Mathematics, 14.12.2021 05:00

Social Studies, 14.12.2021 05:00

Mathematics, 14.12.2021 05:00

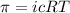
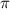 = osmotic pressure of the solution = 0.118 atm
= osmotic pressure of the solution = 0.118 atm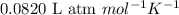
![25^oC=[273+25]=298K](/tpl/images/0466/1252/6a9f9.png)