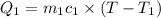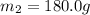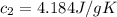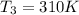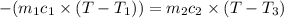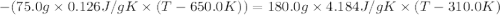Chemistry, 22.01.2020 20:31 justin5163
A75.0 g piece of gold at 650. k is dropped into 180. g of h2o(l) at 310. k in an insulated container at 1 bar pres- sure. calculate the temperature of the system once equilib- rium has been reached. assume that cp, m for au and h2o is constant at their values for 298 k throughout the temperature range of interest.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2
You know the right answer?
A75.0 g piece of gold at 650. k is dropped into 180. g of h2o(l) at 310. k in an insulated container...
Questions


Mathematics, 04.11.2020 04:50




Computers and Technology, 04.11.2020 04:50

Mathematics, 04.11.2020 04:50

History, 04.11.2020 04:50

Mathematics, 04.11.2020 04:50




English, 04.11.2020 04:50



English, 04.11.2020 04:50

Mathematics, 04.11.2020 04:50

Mathematics, 04.11.2020 04:50


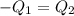
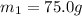
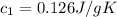
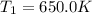
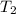 =T
=T