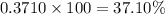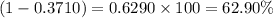Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1


Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
The element iridium exists in nature as two isotopes: 191ir has a mass of 190.9606 u, and 193ir has...
Questions

Mathematics, 02.10.2021 22:00

Mathematics, 02.10.2021 22:00


History, 02.10.2021 22:00


Social Studies, 02.10.2021 22:00

Biology, 02.10.2021 22:00

Geography, 02.10.2021 22:00

Social Studies, 02.10.2021 22:00

Biology, 02.10.2021 22:00

Biology, 02.10.2021 22:00



Social Studies, 02.10.2021 22:00


English, 02.10.2021 22:00

Mathematics, 02.10.2021 22:00


Chemistry, 02.10.2021 22:00

Mathematics, 02.10.2021 22:00

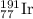 and
and 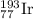 isotopes are 37.10% and 62.90% respectively.
isotopes are 37.10% and 62.90% respectively.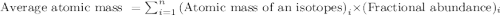 .....(1)
.....(1)![192.22=[(190.9606\times x)+(192.9629\times (1-x))]\\\\x=0.3710](/tpl/images/0466/0782/c8fc1.png)