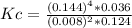Chemistry, 22.01.2020 22:31 Shamplo8817
Steam reforming of methane ( ch4 ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 125l tank with 20 mol of methane gas and 10 mol of water vapor at 38 degrees celsius. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of hydrogen gas to be 18 mol . calculate the concentration equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to significant digits.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1

Chemistry, 23.06.2019 05:00
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1
You know the right answer?
Steam reforming of methane ( ch4 ) produces "synthesis gas," a mixture of carbon monoxide gas and hy...
Questions

English, 07.01.2020 23:31

History, 07.01.2020 23:31






Geography, 07.01.2020 23:31

Mathematics, 07.01.2020 23:31

History, 07.01.2020 23:31


Mathematics, 07.01.2020 23:31


Mathematics, 07.01.2020 23:31

Mathematics, 07.01.2020 23:31

Computers and Technology, 07.01.2020 23:31


English, 07.01.2020 23:31

Chemistry, 07.01.2020 23:31

![Kc = \frac{[H_2]^4*[CO_2]}{[H_2O]^2*[CH_4]}](/tpl/images/0466/2862/a2afc.png)