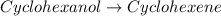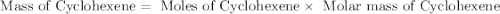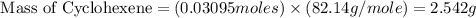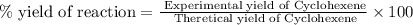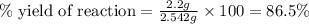Consider the reaction of cyclohexanol and an acid catalyst to obtain cyclohexene.
cyclohexanol (100.16 g/mol) --> cyclohexene (82.14 g/mol)
a reaction was performed in which 3.1 g of cyclohexanol was reacted with an acid catalyst to obtain 2.2 g of cyclohexene. calculate the percent yield for this reaction (in %).

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1
You know the right answer?
Consider the reaction of cyclohexanol and an acid catalyst to obtain cyclohexene.
cycloh...
cycloh...
Questions

Health, 30.07.2019 00:10

Mathematics, 30.07.2019 00:10


Mathematics, 30.07.2019 00:10

Biology, 30.07.2019 00:10



Mathematics, 30.07.2019 00:10







Advanced Placement (AP), 30.07.2019 00:10

Mathematics, 30.07.2019 00:10

Mathematics, 30.07.2019 00:10



English, 30.07.2019 00:10