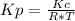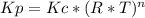Chemistry, 23.01.2020 03:31 bakaoffire
Consider the following chemical equilibrium: c(s) +2h2 (g) ch4 (g) now write an equation below that shows how to calculate from for this reaction at an absolute temperature . you can assume is comfortably above room temperature. if you include any common physical constants in your equation be sure you use their standard symbols, found in the aleks calculator.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
You know the right answer?
Consider the following chemical equilibrium: c(s) +2h2 (g) ch4 (g) now write an equation below that...
Questions




Mathematics, 25.07.2021 21:50

Law, 25.07.2021 21:50




Mathematics, 25.07.2021 21:50

Chemistry, 25.07.2021 21:50




Mathematics, 25.07.2021 21:50



Physics, 25.07.2021 21:50

Mathematics, 25.07.2021 21:50

Mathematics, 25.07.2021 21:50