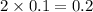Chemistry, 23.01.2020 18:31 danielacortevpe3i66
Raoult's law accounts for the fact that the vapor pressure of a solvent will decrease as the mole fraction of the solvent is decreased. in considering the mole fraction, it is important to consider the total moles of dissolved particles. remember: a particle can be a dissolved molecule or ion. which aqueous solutions would have the lowest vapor pressure

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
You know the right answer?
Raoult's law accounts for the fact that the vapor pressure of a solvent will decrease as the mole fr...
Questions

Mathematics, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00

Chemistry, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00

Business, 12.12.2020 16:00

Social Studies, 12.12.2020 16:00


Chemistry, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00



Mathematics, 12.12.2020 16:00


Mathematics, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00

Social Studies, 12.12.2020 16:00

History, 12.12.2020 16:00

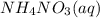 , 0.1 M
, 0.1 M 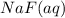 , 0.1 M
, 0.1 M 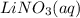 , 0.1 M
, 0.1 M 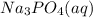 and 0.1 M
and 0.1 M 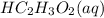
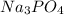
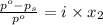
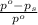 = relative lowering in vapor pressure
= relative lowering in vapor pressure
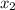 = mole fraction of solute
= mole fraction of solute 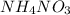
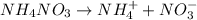
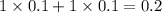
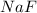
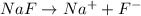
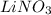
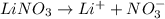
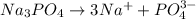
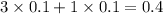
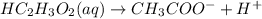 [/tex]
[/tex]