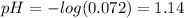In a hospital laboratory, a 10.0 ml sample of gastric juice (predominantly hcl), obtained several hours after a meal, was titrated with 0.1 m naoh to neutrality; 7.2 ml of naoh was required. the patient’s stomach contained no ingested food or drink, thus assume that no buffers were present. what was the ph of the gastric juice?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1


Chemistry, 23.06.2019 18:00
If cos x =sin(20 + x) and 0 < x < 90, the value of x is > 3 , 35 , 350answer is 35, free 50 points
Answers: 1
You know the right answer?
In a hospital laboratory, a 10.0 ml sample of gastric juice (predominantly hcl), obtained several ho...
Questions

Mathematics, 27.06.2019 04:00






Social Studies, 27.06.2019 04:00




English, 27.06.2019 04:00

Mathematics, 27.06.2019 04:00

English, 27.06.2019 04:00

Health, 27.06.2019 04:00


Chemistry, 27.06.2019 04:00

Mathematics, 27.06.2019 04:00




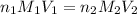
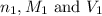 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 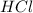
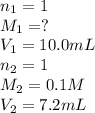
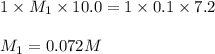
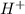 = 0.072
= 0.072![pH=-log[H^+]](/tpl/images/0467/4975/15713.png)