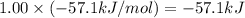Chemistry, 23.01.2020 20:31 powellkolbie
Hcl(aq)+naoh(aq)→nacl(aq)+h2o(l)δh° =−57.1kj/molrxn
the chemical equation above represents the reaction between hcl(aq) and naoh(aq). when equal volumes of 1.00mhcl(aq) and 1.00mnaoh(aq) are mixed, 57.1kj of heat is released. if the experiment is repeated with 2.00mhcl(aq), how much heat would be released?

Answers: 3
Another question on Chemistry

Chemistry, 20.06.2019 18:04
The air exhaled from the lungs of a smoker has a concentration of 24 ppm co. express the concentration as a percent.
Answers: 1


Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1
You know the right answer?
Hcl(aq)+naoh(aq)→nacl(aq)+h2o(l)δh° =−57.1kj/molrxn
the chemical equation above represents th...
the chemical equation above represents th...
Questions

Mathematics, 25.08.2021 20:10


Engineering, 25.08.2021 20:10

Health, 25.08.2021 20:10

Mathematics, 25.08.2021 20:10

Health, 25.08.2021 20:10


Mathematics, 25.08.2021 20:10

Mathematics, 25.08.2021 20:20


Biology, 25.08.2021 20:20


Mathematics, 25.08.2021 20:20

History, 25.08.2021 20:20


Mathematics, 25.08.2021 20:20

Mathematics, 25.08.2021 20:20


Mathematics, 25.08.2021 20:20

Mathematics, 25.08.2021 20:20

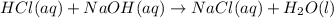 ΔH°=-57.1kJ/mol
ΔH°=-57.1kJ/mol of NaOH
of NaOH