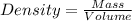To save time you can approximate the initial volume of water to ±1 ml and the initial mass of the solid to ±1 g. for example, if you are asked to add 23 ml of water, add between 22 ml and 24 ml. which metals in each of the following sets will have equal density?
1. 20.2 g gold placed in 21.6 ml of water and 12.0 g copper placed in 21.6 ml of water.
2. 20.2 g silver placed in 21.6 ml of water and 12.0 g silver placed in 21.6 ml of water.
3. 15.2 g copper placed in 21.6 ml of water and 50.0 g copper placed in 23.4 ml of water.
4. 15.4 g gold placed in 20.0 ml of water and 15.7 g silver placed in 20.0 ml of water.
5. 20.2 g silver placed in 21.6 ml of water and 20.2 g copper placed in 21.6 ml of water.
6. 11.2 g gold placed in 21.6 ml of water and 14.9 g gold placed in 23.4 ml of water.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1


Chemistry, 23.06.2019 15:30
Select the correct answer.the gas in a sealed container has an absolute pressure of 125.4 kilopascals. if the air around the container is at a pressure of 99.8 kilopascals, what is thegauge pressure inside the container?
Answers: 3
You know the right answer?
To save time you can approximate the initial volume of water to ±1 ml and the initial mass of the so...
Questions



Mathematics, 06.11.2020 21:10

Mathematics, 06.11.2020 21:10

Chemistry, 06.11.2020 21:10

Mathematics, 06.11.2020 21:10



Chemistry, 06.11.2020 21:10

Mathematics, 06.11.2020 21:10


Health, 06.11.2020 21:10

Physics, 06.11.2020 21:10

Biology, 06.11.2020 21:10


Mathematics, 06.11.2020 21:10

Health, 06.11.2020 21:10


Mathematics, 06.11.2020 21:10

Mathematics, 06.11.2020 21:10