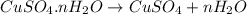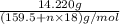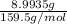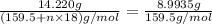Copper(ii) sulfate forms a bright blue hydrate with the formula cuso 4 ⋅ n h 2 o ( s ) . if this hydrate is heated to a high enough temperature, h 2 o ( g ) can be driven off, leaving the grey‑white anhydrous salt cuso 4 ( s ) . a 14.220 g sample of the hydrate was heated to 300 ∘ c . the resulting cuso 4 ( s ) had a mass of 8.9935 g . calculate the val

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2


Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

You know the right answer?
Copper(ii) sulfate forms a bright blue hydrate with the formula cuso 4 ⋅ n h 2 o ( s ) . if this hyd...
Questions

Mathematics, 09.07.2019 01:00


Biology, 09.07.2019 01:00


Mathematics, 09.07.2019 01:00


Mathematics, 09.07.2019 01:00

Geography, 09.07.2019 01:00

Mathematics, 09.07.2019 01:00

Physics, 09.07.2019 01:00



Mathematics, 09.07.2019 01:00

Mathematics, 09.07.2019 01:00

Biology, 09.07.2019 01:00



Biology, 09.07.2019 01:00

Mathematics, 09.07.2019 01:00

Mathematics, 09.07.2019 01:00

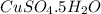 .
.