
Chemistry, 23.01.2020 22:31 ashtonlauber95
Suppose 0.10 mol of cu(no_3)_2 and 1.50 mol of nh_3 are dissolved in water and diluted to a total volume of 1.00 l. calculate the concentrations of cu(nh_3)_4^2+ and of cu^2+ at equilibrium.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3

Chemistry, 23.06.2019 09:00
Water is a highly important natural resource. which of these would be the best method to conserve water? a) drinking bottled water b) monitoring the ph of rivers c) treating and re-using wastewater d) testing nitrate levels in groundwater
Answers: 1
You know the right answer?
Suppose 0.10 mol of cu(no_3)_2 and 1.50 mol of nh_3 are dissolved in water and diluted to a total vo...
Questions






Mathematics, 31.07.2019 00:10


History, 31.07.2019 00:10


History, 31.07.2019 00:10




Mathematics, 31.07.2019 00:10

Mathematics, 31.07.2019 00:10

History, 31.07.2019 00:10

Mathematics, 31.07.2019 00:20


Computers and Technology, 31.07.2019 00:20

Computers and Technology, 31.07.2019 00:20

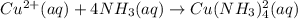
![K_{f} = \frac{[Cu(NH3)^{2+}_{4}]}{[Cu^{2+}][NH_{3}]_{4}}](/tpl/images/0467/9077/53418.png)
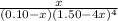
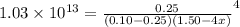
![[NH_{3}] = 1.50 - 4x = (\frac{2.33}{1.03 \times 10^{13}})^{\frac{1}{4}](/tpl/images/0467/9077/925c0.png)
![[Cu(NH_{3})^{2+}_{4}]](/tpl/images/0467/9077/dbe3c.png)
![\frac{1.50 - 2.31284 \times 10{-4}}{4}]](/tpl/images/0467/9077/19dce.png)
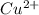 ) is 0.37491425 M.
) is 0.37491425 M.

