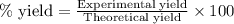During studies of the reaction below,
2 n2h4(l) + n2o4(l) ? 3 n2(g) + 4 h2o(g)
a chemical engineer measured a less-than-expected yield of n2 and discovered that the following side reaction occurs.
n2h4(l) + 2 n2o4(l) ? 6 no(g) + 2 h2o(g)
in one experiment, 11.5 g of no formed when 102.1 g of each reactant was used.
what is the highest percent yield of n2 that can be expected?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
During studies of the reaction below,
2 n2h4(l) + n2o4(l) ? 3 n2(g) + 4 h2o(g)
a chem...
2 n2h4(l) + n2o4(l) ? 3 n2(g) + 4 h2o(g)
a chem...
Questions


Mathematics, 04.10.2019 21:30


Health, 04.10.2019 21:30

Computers and Technology, 04.10.2019 21:30


Mathematics, 04.10.2019 21:30


Mathematics, 04.10.2019 21:30

Mathematics, 04.10.2019 21:30

Biology, 04.10.2019 21:30


History, 04.10.2019 21:30

Mathematics, 04.10.2019 21:30






Advanced Placement (AP), 04.10.2019 21:30

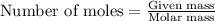 .....(1)
.....(1)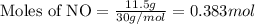
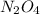 :
: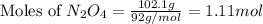
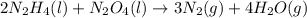 ......(2)
......(2)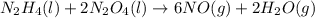 .......(3)
.......(3)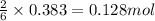 of
of 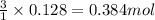 of nitrogen gas
of nitrogen gas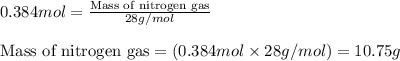
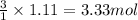 of nitrogen gas
of nitrogen gas