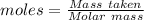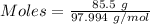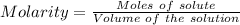Chemistry, 24.01.2020 02:31 andrewlawton8125
Aconcentrated phosphoric acid solution is 85.5% h_3po_4 by mass and has a density of 1.69 g/ml at 25 degree c. what is the molarity of h_3po_4? a. 14.7 m b 0.166 c. 5.16 m d. 19.4 m e. 0.0516 m

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3


Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
You know the right answer?
Aconcentrated phosphoric acid solution is 85.5% h_3po_4 by mass and has a density of 1.69 g/ml at 25...
Questions



Chemistry, 26.07.2019 02:00






English, 26.07.2019 02:00




Biology, 26.07.2019 02:00

Health, 26.07.2019 02:00




Biology, 26.07.2019 02:00


Mathematics, 26.07.2019 02:00

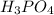 is 85.5 % w/w in solution.
is 85.5 % w/w in solution.