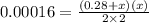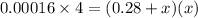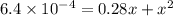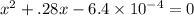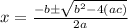Chemistry, 24.01.2020 10:31 matthewquattlebum
When solid nh4hs and 0.28 mol nh3(g) were placed in a 2 l vessel at 24◦c, the equilibriumnh4hs(s)⇀↽nh3(g) + h2s(g)for which kc= 0.00016, was reached. what is the equilibrium concentration of nh3? answer in units of mol/l

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
When solid nh4hs and 0.28 mol nh3(g) were placed in a 2 l vessel at 24◦c, the equilibriumnh4hs(s)⇀↽n...
Questions

Biology, 29.11.2020 21:50

History, 29.11.2020 21:50



Mathematics, 29.11.2020 21:50


Mathematics, 29.11.2020 21:50



Mathematics, 29.11.2020 21:50



Biology, 29.11.2020 21:50

Arts, 29.11.2020 21:50


Mathematics, 29.11.2020 21:50


Mathematics, 29.11.2020 21:50

Mathematics, 29.11.2020 21:50

English, 29.11.2020 21:50

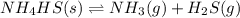
![Kc=[NH_{3}][H_{2}S]](/tpl/images/0468/7808/2a772.png)
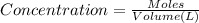
![[NH_{3}]=\frac{0.28 +x}{2}](/tpl/images/0468/7808/aa8ed.png)
![[H_{2}S]=\frac{x}{2}](/tpl/images/0468/7808/ba461.png)