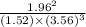Chemistry, 24.01.2020 10:31 cheervolley
A1.000 l vessel is filled with 1.000 mole of n2,2.000 moles of h2, and 3.000 moles of nh3. when the reaction n2(g) + 3 h2(g)⇀↽2 nh3(g) comes to equilibrium, it is observed that the concentration of nh3is 2.12 moles/l. what is the numerical value of the equilibrium constant kc?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1
You know the right answer?
A1.000 l vessel is filled with 1.000 mole of n2,2.000 moles of h2, and 3.000 moles of nh3. when the...
Questions

Mathematics, 02.08.2019 08:30


Mathematics, 02.08.2019 08:30

Mathematics, 02.08.2019 08:30

Mathematics, 02.08.2019 08:30



Physics, 02.08.2019 08:30

Mathematics, 02.08.2019 08:30


History, 02.08.2019 08:30

Arts, 02.08.2019 08:30

Physics, 02.08.2019 08:30


Chemistry, 02.08.2019 08:30


Chemistry, 02.08.2019 08:30



![[NH^3]= \frac{3mol}{1l}](/tpl/images/0468/7747/69ad7.png) = 3M
= 3M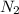 ] = 1M
] = 1M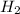 ] = 2 M
] = 2 M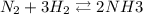
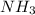 ] = 1.96 M
] = 1.96 M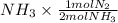 ) = 0.52 mol created (in addition to 1 mol already in vessel)
) = 0.52 mol created (in addition to 1 mol already in vessel)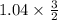 1.56 moles created
1.56 moles created 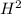 ] = 1.56 + 2 = 3.56 M
] = 1.56 + 2 = 3.56 M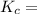
![\frac{[NH^3]^2}{N^2[H^2]^3}](/tpl/images/0468/7747/7f945.png)