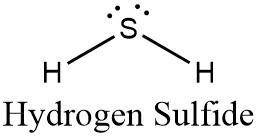Chemistry, 24.01.2020 17:31 elysalmeron05
1point
which statement best describes the polarity of the molecule h, s?
a. it is polar, because the bond polarities add together in a linear
molecule.
b. it is nonpolar, because the bond polarities cancel each other out in
a linear molecule.
o
c. it is polar, because the bond polarities add together in a bent
molecule.
o
d. it is nonpolar, because the bonds between hydrogen (h) and sulfur
(s) are not polar.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1


Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

You know the right answer?
1point
which statement best describes the polarity of the molecule h, s?
a. it is polar...
which statement best describes the polarity of the molecule h, s?
a. it is polar...
Questions



Mathematics, 07.07.2020 14:01


Physics, 07.07.2020 14:01



Mathematics, 07.07.2020 14:01

Physics, 07.07.2020 14:01



Engineering, 07.07.2020 15:01

Mathematics, 07.07.2020 15:01


Biology, 07.07.2020 15:01




Mathematics, 07.07.2020 15:01