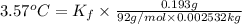Chemistry, 24.01.2020 18:31 Braxtonw875
1) if 0.193 grams of toluene is dissolved in 2.532 grams of p-xylene, what is the molality of toluene in the solution? 2) if a freezing point depression of 3.57°celcius is measured for the solution described in question 1, calculate  for p-xylene.3) suppose you dissolved 0.123 gram of pentane in 2.493 grams of p-xylene and measured a freezing point depression of 2.88°celcius for the solution. calculate the molar mass of pentane using this data and the value for
for p-xylene.3) suppose you dissolved 0.123 gram of pentane in 2.493 grams of p-xylene and measured a freezing point depression of 2.88°celcius for the solution. calculate the molar mass of pentane using this data and the value for  that you calculated in question 2.
that you calculated in question 2.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:20
Why is an elements atomic mass not listed as a whole number on the periodic table
Answers: 2


Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1
You know the right answer?
1) if 0.193 grams of toluene is dissolved in 2.532 grams of p-xylene, what is the molality of toluen...
Questions

Mathematics, 14.07.2019 16:00







Mathematics, 14.07.2019 16:00

Mathematics, 14.07.2019 16:00

History, 14.07.2019 16:00

English, 14.07.2019 16:00


History, 14.07.2019 16:00



Mathematics, 14.07.2019 16:00

Biology, 14.07.2019 16:00


Computers and Technology, 14.07.2019 16:00

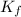 for xylene is 4.309°C/m.
for xylene is 4.309°C/m.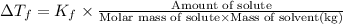
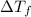 =depression in freezing point
=depression in freezing point