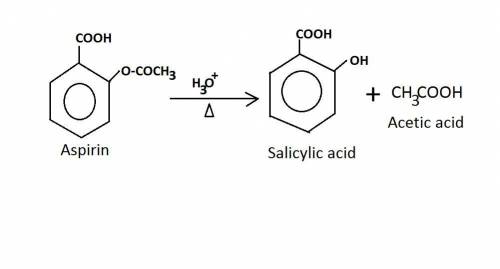
Old aspirin exposed to moisture often smells like acetic acid (vinegar). when aspirin is heated in boiling water, it decomposes and gives off a vinegar smell. the resulting solution gives a positive fecl3 test. why is this test positive? ( explain in detail! )
write the chemical equation for the reaction of aspirin and water at high temperature.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2

Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1
You know the right answer?
Old aspirin exposed to moisture often smells like acetic acid (vinegar). when aspirin is heated in b...
Questions



Mathematics, 08.03.2021 19:40

Mathematics, 08.03.2021 19:40




Mathematics, 08.03.2021 19:40

Mathematics, 08.03.2021 19:40


Geography, 08.03.2021 19:40






Mathematics, 08.03.2021 19:40



Mathematics, 08.03.2021 19:40

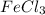 .
.