Chemistry, 24.01.2020 22:31 freshysans4
The freezing point of an aqueous solution containing an unknown solute is -2.60 degc. the solution was prepared by dissolving 5.00 g of a nonelectrolytic solute in 100. ml of water. what is the molar mass of the unknown solute?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1

Chemistry, 23.06.2019 08:30
Kelly has come up with an explanation for why her sister is sometimes in a good mood and other times in a bad mood. she speculates that it is based on the hours of sleep her sister got the previous night. this explanation for her sister's behaviors is an example of a(n)
Answers: 3

Chemistry, 23.06.2019 15:30
What is the predicted change in the boiling point of water when 1.50 g of barium chloride is dissolved in 1.50 kg of water?
Answers: 3
You know the right answer?
The freezing point of an aqueous solution containing an unknown solute is -2.60 degc. the solution w...
Questions



Mathematics, 17.10.2021 14:00


Mathematics, 17.10.2021 14:00









Mathematics, 17.10.2021 14:00



Mathematics, 17.10.2021 14:00


Mathematics, 17.10.2021 14:00

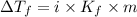
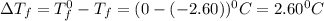 = Depression in freezing point
= Depression in freezing point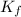 = freezing point constant =
= freezing point constant = 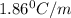
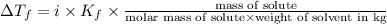
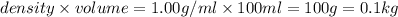 (1kg=1000g)
(1kg=1000g)