
Chemistry, 25.01.2020 01:31 stupidsmoke4272
In a photoelectric effect experiment, electrons are ejected from a titanium surface (work function, 3 ev) following irradiation with uv light. the energy of the incident uv light is 7.2 x 10-19 j.
(a) calculate the wavelength of the ejected electrons.
(b) calculate the wavelength of the incident uv light.
(c) would an iron surface (ф-4.7 ev require a longer or shorter wavelength of light to eject electrons with the same wavelength calculated in part (a)? briefly explain.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:10
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
In a photoelectric effect experiment, electrons are ejected from a titanium surface (work function,...
Questions

Mathematics, 01.08.2019 11:00

World Languages, 01.08.2019 11:00

Health, 01.08.2019 11:00


History, 01.08.2019 11:00

English, 01.08.2019 11:00


History, 01.08.2019 11:00



Biology, 01.08.2019 11:00


Advanced Placement (AP), 01.08.2019 11:00


Advanced Placement (AP), 01.08.2019 11:00

Computers and Technology, 01.08.2019 11:00

Mathematics, 01.08.2019 11:00

Advanced Placement (AP), 01.08.2019 11:00


Mathematics, 01.08.2019 11:00

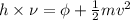
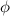 = work function
= work function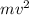 = kinetic energy of electron
= kinetic energy of electron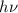 is
is 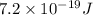 . And,
. And, 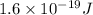
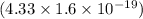 J
J  J
J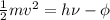
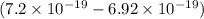 J
J J
J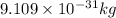 .
. 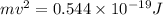
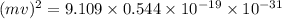


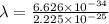
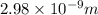
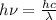
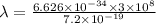
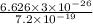
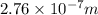
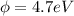
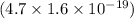 J
J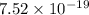 J
J


