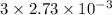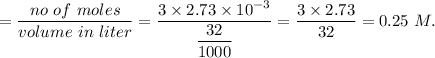Chemistry, 25.01.2020 02:31 Ayyyyeeeeeeewuzgud
A32.00 ml sample of an unknown h3po4 solution is titrated with a 0.110 m naoh solution. the equivalence point is reached when 24.83 ml of naoh solution is added. what is the concentration of the unknown h3po4 solution? the neutralization reaction is
h3po4(aq)+3naoh(aq)→3h2o(l)+na3po4( aq)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3
You know the right answer?
A32.00 ml sample of an unknown h3po4 solution is titrated with a 0.110 m naoh solution. the equivale...
Questions





Arts, 10.03.2021 01:00


Mathematics, 10.03.2021 01:00




English, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00


Computers and Technology, 10.03.2021 01:00

Chemistry, 10.03.2021 01:00



Biology, 10.03.2021 01:00

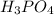 in sample is 0.25 M.
in sample is 0.25 M.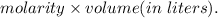
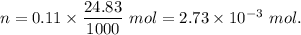
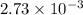 mol of NaOH reacts with
mol of NaOH reacts with