Chemistry, 25.01.2020 04:31 tsmalls70988
Given the reactions below, answer the following questions.
cl_2(g) + f_2(g) rlhar 2clf(g) delta g degree_rxn = 115.4 kj/mol
cl_2(g) + br_2(g) rlhar 2clbr(g) delta g degree_rxn = -2.0 kj/mol
calculate the delta g degree_rxn for 2clf(g) + br_2(g) rlhar 2clbr(g) + f_2(g) kj/mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1
You know the right answer?
Given the reactions below, answer the following questions.
cl_2(g) + f_2(g) rlhar 2clf(g) del...
cl_2(g) + f_2(g) rlhar 2clf(g) del...
Questions


Mathematics, 16.07.2021 22:30

Mathematics, 16.07.2021 22:30

Health, 16.07.2021 22:30


Mathematics, 16.07.2021 22:30



Mathematics, 16.07.2021 22:30

Mathematics, 16.07.2021 22:30

Mathematics, 16.07.2021 22:30



Mathematics, 16.07.2021 22:30

Mathematics, 16.07.2021 22:30

Mathematics, 16.07.2021 22:30

Mathematics, 16.07.2021 22:30



Mathematics, 16.07.2021 22:30

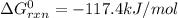
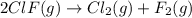 ;
; 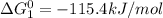
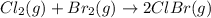 ;
; 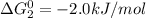
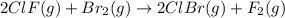 ;
;