
Chemistry, 27.01.2020 01:31 eagles2286
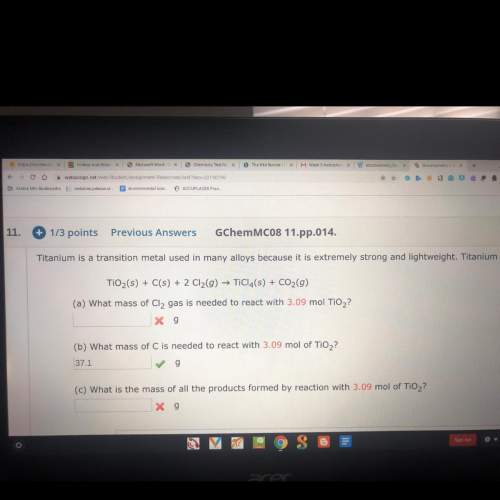
Titanium is a transition metal used in many alloys because it is extremely strong and lightweight. titanium tetrachloride (ticl4) is extracted from titanium oxide using chloride and coke (carbon).
a) what mass of cl2 gas is needed to react with 3.09 mol tio2?
c) what is the mass of all the products formed by reaction with 3.09 mol tio2

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
You know the right answer?
Titanium is a transition metal used in many alloys because it is extremely strong and lightweight. t...
Questions




Mathematics, 12.07.2019 16:00

French, 12.07.2019 16:00


Mathematics, 12.07.2019 16:00

Biology, 12.07.2019 16:00


Social Studies, 12.07.2019 16:00

Advanced Placement (AP), 12.07.2019 16:00

Social Studies, 12.07.2019 16:00

English, 12.07.2019 16:00

Mathematics, 12.07.2019 16:00

History, 12.07.2019 16:00


Chemistry, 12.07.2019 16:00


Biology, 12.07.2019 16:00

Chemistry, 12.07.2019 16:00



