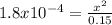Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 04:00
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1
You know the right answer?
Calculate the ph for the following weak acid.
a solution of hcooh has 0.15m hcooh at equ...
a solution of hcooh has 0.15m hcooh at equ...
Questions


Chemistry, 06.10.2019 17:30



Health, 06.10.2019 17:30


History, 06.10.2019 17:30

Biology, 06.10.2019 17:30

Biology, 06.10.2019 17:30


Mathematics, 06.10.2019 17:30


Mathematics, 06.10.2019 17:30

Mathematics, 06.10.2019 17:30



Mathematics, 06.10.2019 17:30



Mathematics, 06.10.2019 17:30

![ka = \frac{[H3O][HCOO]}{[HCOOH]}](/tpl/images/0471/8959/57b44.png)
![1.8 x 10^{-4} = \frac{[x][x]}{0.15 - x}](/tpl/images/0471/8959/c09c0.png)