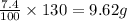Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
Asolid mixture consists of 47.6g of kno3 (potassium nitrate) and 8.4g of k2so4 (potassium sulfate)....
Questions


Mathematics, 28.09.2019 19:50

Mathematics, 28.09.2019 19:50

Chemistry, 28.09.2019 19:50



Mathematics, 28.09.2019 19:50




Social Studies, 28.09.2019 19:50


Mathematics, 28.09.2019 19:50

Mathematics, 28.09.2019 19:50

Chemistry, 28.09.2019 19:50



Mathematics, 28.09.2019 19:50