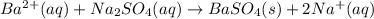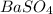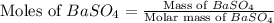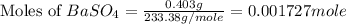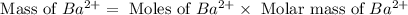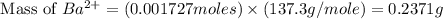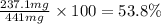Chemistry, 27.01.2020 21:31 Deliriousg636
Achemist added an excess of sodium sulfate to a solution of a soluble barium compound to precipitate all of the barium ion as barium sulfate, baso4. how many grams of barium ion are in a 441-mg sample of the barium compound if a solution of the sample gave 403 mg baso4 precipitate? what is the mass percentage of barium in the compound?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:20
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
Achemist added an excess of sodium sulfate to a solution of a soluble barium compound to precipitate...
Questions

Mathematics, 25.02.2020 05:11


English, 25.02.2020 05:12







Computers and Technology, 25.02.2020 05:12


History, 25.02.2020 05:12