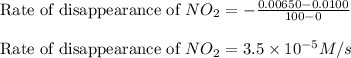Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
You know the right answer?
Nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: 2no2 → 2no + o2 in a parti...
Questions

Chemistry, 18.12.2020 01:00



Mathematics, 18.12.2020 01:00



Mathematics, 18.12.2020 01:00



Social Studies, 18.12.2020 01:00


Social Studies, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00

Computers and Technology, 18.12.2020 01:00




Mathematics, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00

History, 18.12.2020 01:00

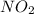 is
is 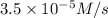
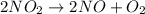
![\text{Rate of disappearance of }NO_2=-\frac{\Delta [NO_2]}{\Delta t}](/tpl/images/0473/6156/ea698.png)
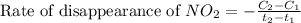
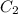 = final concentration of
= final concentration of 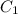 = initial concentration of
= initial concentration of 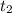 = final time = 100 minutes
= final time = 100 minutes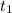 = initial time = 0 minutes
= initial time = 0 minutes