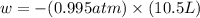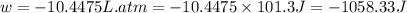Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 06:30
Which of these natural resources is non-renewable a.corn b.wind c.geothermal d.natural gas
Answers: 2

Chemistry, 23.06.2019 07:00
4. glenn andrews recently bought a motorcycle for $3,950. if he had to pay 6% sales tax on the bike, what was the total cost of the motorcycle?
Answers: 1
You know the right answer?
Calculate the expansion work done against a constant external pressure of 0.995 atm and at a constan...
Questions

Mathematics, 18.08.2019 16:30

Social Studies, 18.08.2019 16:30

History, 18.08.2019 16:30


Mathematics, 18.08.2019 16:30

English, 18.08.2019 16:30

Biology, 18.08.2019 16:30

Chemistry, 18.08.2019 16:30


Social Studies, 18.08.2019 16:30


History, 18.08.2019 16:30


History, 18.08.2019 16:30

Mathematics, 18.08.2019 16:30



Mathematics, 18.08.2019 16:30

Mathematics, 18.08.2019 16:30

History, 18.08.2019 16:30

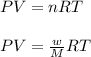
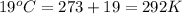
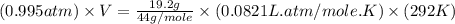
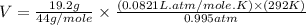
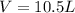
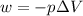
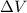 = volume = 10.5 L
= volume = 10.5 L