Chemistry, 28.01.2020 00:31 kyzerlinda
Based on the thermodynamic properties provided for water, determine the amount of energy released for 155.0 g of water to go from 39.0 °c to -36.5°c. property melting point boiling point ahfus ahvap cp (s) value 0.0 100.0 6.01 40.67 37.1 75.3 33.6 units oc kj/mol kj/mol j/mol. oc j/mol c mol oc cp (g)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1


Chemistry, 23.06.2019 06:30
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1
You know the right answer?
Based on the thermodynamic properties provided for water, determine the amount of energy released fo...
Questions

Mathematics, 24.08.2019 18:20


History, 24.08.2019 18:20

Mathematics, 24.08.2019 18:20

Health, 24.08.2019 18:20

Biology, 24.08.2019 18:20



Mathematics, 24.08.2019 18:20




World Languages, 24.08.2019 18:20

Mathematics, 24.08.2019 18:20

Mathematics, 24.08.2019 18:20

Geography, 24.08.2019 18:20


Chemistry, 24.08.2019 18:20


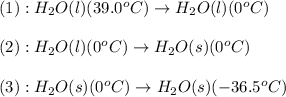
![\Delta H=[m\times c_{p,l}\times (T_{final}-T_{initial})]+m\times (-\Delta H_{fusion})+[m\times c_{p,l}\times (T_{final}-T_{initial})]](/tpl/images/0474/1272/1181a.png)
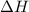 = heat available for the reaction = ?
= heat available for the reaction = ?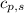 = specific heat of solid water =
= specific heat of solid water = 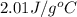
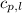 = specific heat of liquid water =
= specific heat of liquid water = 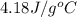
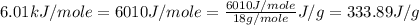
![\Delta H=[155.0g\times 4.18J/g^oC\times (0-(39.0))^oC]+155.0g\times -333.89J/g+[155.0g\times 2.01J/g^oC\times (-36.5-0)^oC]](/tpl/images/0474/1272/a7829.png)