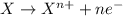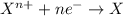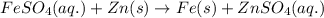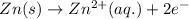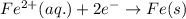Chemistry, 28.01.2020 00:31 leianagaming
Study this chemical reaction: feso4 (aq) + zn (s) --> fe (s) + znso4 (aq) then, write balanced half-reactions describing the oxidation and reduction that happen in this reaction.
oxidation:
reduction:

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
Study this chemical reaction: feso4 (aq) + zn (s) --> fe (s) + znso4 (aq) then, write balanced...
Questions


Chemistry, 19.11.2019 17:31


Mathematics, 19.11.2019 17:31

History, 19.11.2019 17:31



Mathematics, 19.11.2019 17:31



Social Studies, 19.11.2019 17:31


Mathematics, 19.11.2019 17:31




History, 19.11.2019 17:31


Geography, 19.11.2019 17:31