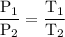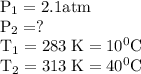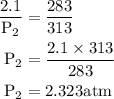Chemistry, 28.01.2020 01:31 datboyjulio21
At 10.°c, 20.g of oxygen gas exerts a pressure of 2.1atm in a rigid, 7.0l cylinder. assuming ideal behavior, if the temperature of the gas was raised to 40.°c, which statement indicates the new pressure and explains why?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
At 10.°c, 20.g of oxygen gas exerts a pressure of 2.1atm in a rigid, 7.0l cylinder. assuming ideal b...
Questions




Mathematics, 16.10.2020 20:01




History, 16.10.2020 20:01

History, 16.10.2020 20:01



Mathematics, 16.10.2020 20:01

English, 16.10.2020 20:01


Mathematics, 16.10.2020 20:01

Spanish, 16.10.2020 20:01

Mathematics, 16.10.2020 20:01

Mathematics, 16.10.2020 20:01


 = 2.323 atm.
= 2.323 atm.  or temperature of oxygen = 10-degrees celciusPressure or P
or temperature of oxygen = 10-degrees celciusPressure or P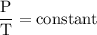 Likewise,
Likewise,