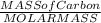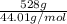Chemistry, 28.08.2019 01:00 ashleyremon
The molar mass of carbon dioxide (co2) is 44.01 g/mol. the molar mass of water (h2o) is 18.01 g/mol. a reaction uses 528 g of co2. how many moles of water are used in this reaction?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 23.06.2019 21:30
Match the following items. match the items in the left column to the items in the right column. 1. general or nonspecific observation best fit curve 2. educated guess quantitative 3. when two variables change in the same direction, one remaining larger than the other by the same factor theory 4. "measured" observation control 5. standard, reference data, or experiment qualitative 6. a presently accepted explanation of how something happens law 7. a statement that explains what something does under the same conditions hypothesis 8. a curve drawn to average the data's deviations direct relationship
Answers: 1
You know the right answer?
The molar mass of carbon dioxide (co2) is 44.01 g/mol. the molar mass of water (h2o) is 18.01 g/mol....
Questions



Mathematics, 11.02.2021 05:20

History, 11.02.2021 05:20




Mathematics, 11.02.2021 05:20

Mathematics, 11.02.2021 05:20




Mathematics, 11.02.2021 05:20


Mathematics, 11.02.2021 05:20

Mathematics, 11.02.2021 05:20

Mathematics, 11.02.2021 05:20


Mathematics, 11.02.2021 05:20