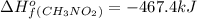Top fuel dragsters and funny cars burn nitro-methane as fuel according to the following balanced combustion equation: 2ch3no2(l)+3/2o2(g)→2co2(g)+3h2o(g) +n2(g). the standard enthalpy of combustion for nitromethane is −709.2kj/mol. calculate the standard enthalpy of formation(delta h formation) for nitro-methane.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
Top fuel dragsters and funny cars burn nitro-methane as fuel according to the following balanced com...
Questions


Mathematics, 28.06.2019 01:30

Mathematics, 28.06.2019 01:30

Social Studies, 28.06.2019 01:30

Mathematics, 28.06.2019 01:30

Social Studies, 28.06.2019 01:30

Mathematics, 28.06.2019 01:30


Mathematics, 28.06.2019 01:30




Social Studies, 28.06.2019 01:30

Social Studies, 28.06.2019 01:30


Social Studies, 28.06.2019 01:30

Mathematics, 28.06.2019 01:30

Biology, 28.06.2019 01:30

Social Studies, 28.06.2019 01:30

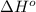
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0475/2966/45485.png)
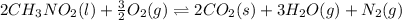
![\Delta H^o_{rxn}=[(n_{(CO_2)}\times \Delta H^o_f_{(CO_2)})+(n_{(H_2O)}\times \Delta H^o_f_{(H_2O)})+(n_{(N_2)}\times \Delta H^o_f_{(N_2)})]-[(n_{(CH_3NO_2)}\times \Delta H^o_f_{(CH_3NO_2)})+(n_{(O_2)}\times \Delta H^o_f_{(O_2)})]](/tpl/images/0475/2966/ea72d.png)
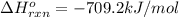
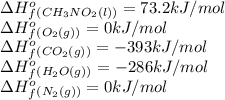
![-709.2=[(2\times -393)+(3\times -286)+(1\times 0)]-[(2\times \Delta H^o_f_{(CH_3NO_2)})+(\frac{3}{2}\times 0)]](/tpl/images/0475/2966/4d16f.png)