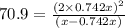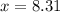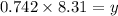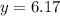Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

You know the right answer?
At particular temperature, kp = 70.9 for the following reactionn2o4(g) == 2no2(g)1. a certain pressu...
Questions


Mathematics, 07.05.2020 01:06

Mathematics, 07.05.2020 01:06



Physics, 07.05.2020 01:06



Mathematics, 07.05.2020 01:06

History, 07.05.2020 01:06


Mathematics, 07.05.2020 01:06


History, 07.05.2020 01:06

Mathematics, 07.05.2020 01:06


Mathematics, 07.05.2020 01:06


Mathematics, 07.05.2020 01:06

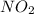 is, 12.34 atm
is, 12.34 atm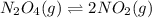
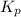 for above reaction follows:
for above reaction follows: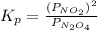 ........(1)
........(1)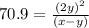 ..............(2)
..............(2)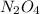 remains at equilibrium. That means,
remains at equilibrium. That means,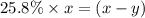
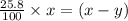
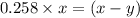
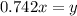 ..............(3)
..............(3)