Asample of 0.900 mol n2o is placed in a sealed
container, where it decomposes irreversibly to...

Chemistry, 28.01.2020 08:31 jessicajose4238
Asample of 0.900 mol n2o is placed in a sealed
container, where it decomposes irreversibly to n2 and o2
in a first-order reaction. after 42.0 min, 0.640 mol n2o
remains. how long will it take for the reaction to be
90.0% complete?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1
You know the right answer?
Questions





Mathematics, 13.07.2021 04:00




Computers and Technology, 13.07.2021 04:00

Mathematics, 13.07.2021 04:00

Mathematics, 13.07.2021 04:00




Health, 13.07.2021 04:00



History, 13.07.2021 04:00

English, 13.07.2021 04:00

Social Studies, 13.07.2021 04:00

![r=-\frac{d[N_2O]}{dt}= k[N_2O]\\ \\ \frac{d[N_2O]}{dt}= -k[N_2O]](/tpl/images/0475/9615/4d9bd.png)
![\frac{d[N_2O]}{[N_2O]}=-kdt\\ \\ ln[N_2O]-ln[N_2O]_0=-kt](/tpl/images/0475/9615/af464.png)
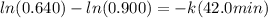 k = 0.00812 min⁻¹
k = 0.00812 min⁻¹

