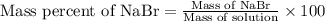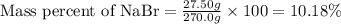Chemistry, 28.01.2020 19:42 zamariahyou
An aqueous nabr solution has a mass of 270.0 g and contains 27.50 g nabr. calculate the mass percent nabr .

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1


Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1
You know the right answer?
An aqueous nabr solution has a mass of 270.0 g and contains 27.50 g nabr. calculate the mass percent...
Questions


Mathematics, 09.06.2021 19:10

English, 09.06.2021 19:10

Mathematics, 09.06.2021 19:10


Mathematics, 09.06.2021 19:10



Mathematics, 09.06.2021 19:10

Mathematics, 09.06.2021 19:10


Biology, 09.06.2021 19:10

History, 09.06.2021 19:10


Mathematics, 09.06.2021 19:10

Mathematics, 09.06.2021 19:10

Arts, 09.06.2021 19:10


Mathematics, 09.06.2021 19:10

Mathematics, 09.06.2021 19:10