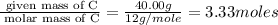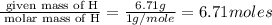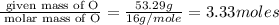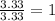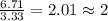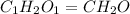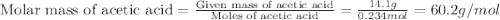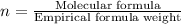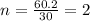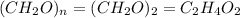Chemistry, 28.01.2020 19:48 kaylallangari549
Acetic acid is an organic compound composed of 40.00% c, 6.71% h, and the rest oxygen. if 0.234 mol of acetic acid has a mass of 14.1 g, what are the empirical and molecular formulas of acetic acid?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1
You know the right answer?
Acetic acid is an organic compound composed of 40.00% c, 6.71% h, and the rest oxygen. if 0.234 mol...
Questions

English, 19.12.2020 21:30

Social Studies, 19.12.2020 21:30










Mathematics, 19.12.2020 21:30




Advanced Placement (AP), 19.12.2020 21:30


English, 19.12.2020 21:30

English, 19.12.2020 21:30

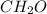 and
and 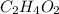 respectively.
respectively.