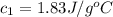Chemistry, 28.01.2020 19:47 sierraseideman1023
A24.7 g sample of beryllium at 96.7°c is placed into 59.1 ml of water at 20.2°c in an insulated container. the temperature of the water at thermal equilibrium is 32.0°c. what is the specific heat of beryllium? assume a density of 1.00 g/ml for water

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:00
One of the cell membrane's functions is to protect the cell keep wastes in the cell create new cells keep light out of the cell
Answers: 1

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
A24.7 g sample of beryllium at 96.7°c is placed into 59.1 ml of water at 20.2°c in an insulated cont...
Questions


Mathematics, 26.06.2019 16:30

Mathematics, 26.06.2019 16:30


Mathematics, 26.06.2019 16:30

Mathematics, 26.06.2019 16:30



History, 26.06.2019 16:30


Mathematics, 26.06.2019 16:30


Mathematics, 26.06.2019 16:30



Mathematics, 26.06.2019 16:30

Mathematics, 26.06.2019 16:30

Biology, 26.06.2019 16:30


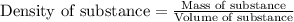
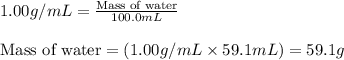
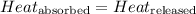
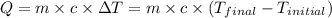
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0478/1893/09236.png) ......(1)
......(1)
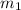 = mass of water = 24.7 g
= mass of water = 24.7 g
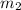 = mass of beryllium= 59.1 g
= mass of beryllium= 59.1 g
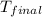 = final temperature = 32.0°C
= final temperature = 32.0°C
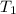 = initial temperature of water = 20.2°C
= initial temperature of water = 20.2°C
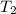 = initial temperature of beryllium =96.7°C
= initial temperature of beryllium =96.7°C
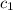 = specific heat of water= 4.186 J/g°C
= specific heat of water= 4.186 J/g°C
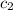 = specific heat of beryllium = ?
= specific heat of beryllium = ?![59.1\times 4.186\times (32.0-20.2)=-[24.7\times c_2\times (32.0-96.7)]](/tpl/images/0478/1893/cca22.png)