
Chemistry, 28.01.2020 20:51 queenkimm26
Assume that each atom is a sphere, and that the surface of each atom is in contact with its nearest neighbor. determine the percentage of unit cell volume that is occupied in (a) a face- centered cubic lattice, (b) a body-centered cubic lattice, and (c) a diamond lattice.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2
You know the right answer?
Assume that each atom is a sphere, and that the surface of each atom is in contact with its nearest...
Questions



Mathematics, 14.12.2020 19:40

Biology, 14.12.2020 19:40

Arts, 14.12.2020 19:40

Mathematics, 14.12.2020 19:40

Mathematics, 14.12.2020 19:40

Mathematics, 14.12.2020 19:40


Mathematics, 14.12.2020 19:40



History, 14.12.2020 19:40


Mathematics, 14.12.2020 19:40



Chemistry, 14.12.2020 19:40

Mathematics, 14.12.2020 19:40

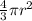
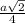
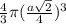 =
= 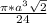
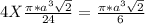
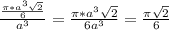 = 0.7405
= 0.7405 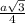
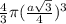 =
=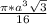
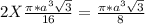
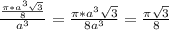 = 0.6803
= 0.6803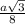
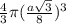 =
= 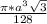
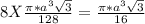
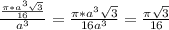 = 0.3401
= 0.3401


